Testing Process
Donor Screening
This is done before delivery to determine eligible donors for the Amniotic Membrane Stem Cell Derived Allograft. We only collect donated tissue from donors who pass the Donor Screening. This is a third-party testing.
Infectious Diseases
Testing
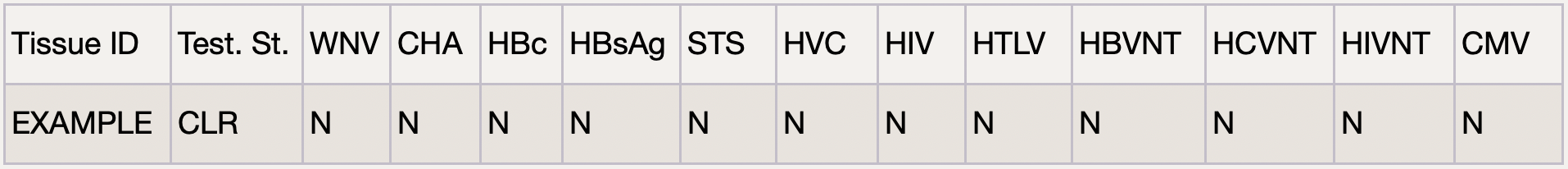
Donor blood tested for 13 different diseases including From HIV to Zika Virus and anything in between. We only process tissue from donors who pass the Infectious Disease Testing. This is also a third-party testing.
Microbial Contamination
We conduct both in-house and third-party microbial testing of our finished product to ensure our products are free from microbial contaminations.
We only sell products that pass the Microbial Contamination Testing.
Particle Counting
We also do a third-party test on our products to count the number of particles present on our products.